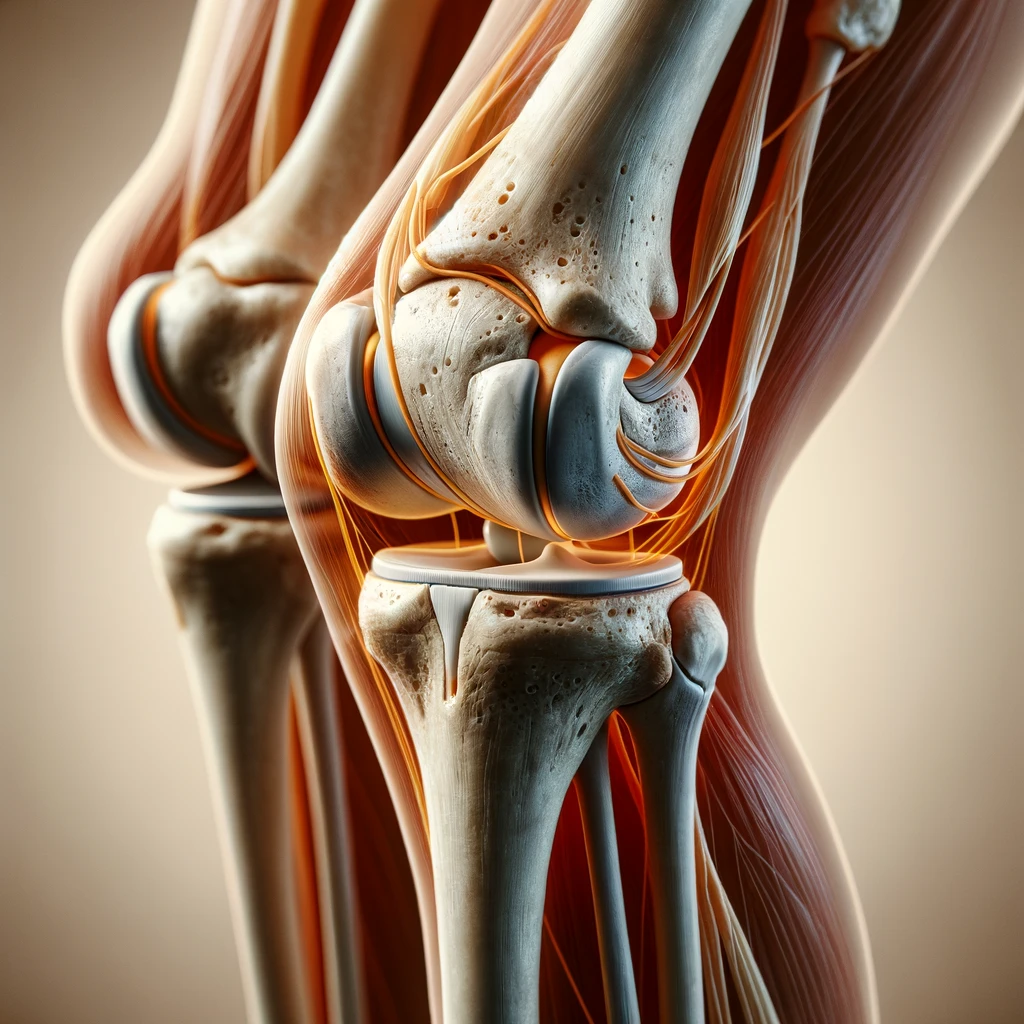
Exactech Replacement Lawsuits Overview
Exactech lawsuits are seeking compensation for injuries stemming from defective hip, knee, and ankle replacements. The company issued recalls for thousands of its medical devices in 2021 and 2022 due to faulty packaging, leading to ongoing litigation.
Latest Updates on Exactech Lawsuits
As of February 2024, there are 1,169 pending lawsuits in multidistrict litigation (MDL) before the U.S. District Court for the Eastern District of New York, presided over by Judge Nicholas G. Garaufis.
No trials have taken place in the MDL yet, and the number of lawsuits continues to rise steadily as new cases are accepted.
Key Updates in Exactech Lawsuits
- In January 2024, the FDA announced the recall of an undetermined number of AcuMatch Hip System units due to complaints of vacuum loss in the inner vacuum bag of four devices, affecting products distributed in the U.S. and 12 other countries.
- A status conference for the Exactech class action MDL is scheduled for March 13, 2024, to address the current status of litigation, unresolved discovery matters, and the selection of bellwether cases.
- Parties involved in the MDL and Exactech litigation in Florida were in the process of selecting bellwether test trials as of December 2023.
- In October 2023, a pending transfer order was lifted, transferring 20 additional cases into MDL 3044, assigned to Senior District Judge Nicholas G. Garaufis in New York.
- In March 2023, Judge Marcia M. Henry ordered all plaintiffs in the MDL to complete Plaintiff Fact Sheets before June 6, 2023.
- Exactech cases from across the country were consolidated into multidistrict litigation in New York MDL 3044 in October 2022.
Past Litigation and Recalls
Exactech previously faced legal issues for providing faulty knee replacements to VA, Medicare, and Medicaid beneficiaries. Additionally, the company settled a lawsuit with the federal government in 2010 for nearly $3 million over false claims submitted to Medicare.
Lawsuits filed after Exactech recalls are based on issues where defective packaging led to premature breakdowns of polyethylene hip and knee inserts, affecting thousands of patients and possibly resulting in complications related to joint replacement surgery.