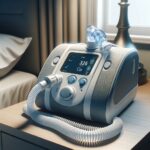
Philips, headquartered in the Netherlands, recently declared that it would discontinue the sale of its CPAP and BiPAP machines for sleep apnea in the United States. This action comes after reaching a settlement with the Food and Drug Administration (FDA) and facing multiple challenges, including recalls and legal disputes.
The recall of millions of devices in 2021, driven by concerns about potentially harmful foam, has sparked discussions about Philips’ market position and its impact on patients. There were worries that the foam used in the devices could degrade over time, posing health risks to users.
Philips is currently grappling with numerous legal challenges from patients who allege health problems associated with using their devices. As of January 2024, close to 800 Philips CPAP lawsuits were pending in multidistrict litigation in a Pennsylvania federal court.
Philips’ Response to Recalls and FDA Settlement Halting sales is a significant move for Philips to comply with the terms of a consent decree following the recall of its CPAP machines due to concerns about foam degradation and potential health risks, including cancer.
To address FDA concerns, Philips plans to enhance its U.S. facilities and refrain from selling new CPAP devices until they meet FDA standards. However, the company will continue to provide service for existing Philips devices in the U.S.
Following the announcement, Philips’ shares experienced an immediate 8.3% decline in Amsterdam. Analysts are skeptical about the company’s ability to regain its position in the competitive U.S. CPAP market, potentially benefiting competitors like ResMed, whose stock saw a nearly 2% rise during early U.S. trading.
The finalization of the consent decree and court approval in the U.S. adds to the uncertainty surrounding Philips’ future timeline.
Ongoing Challenges and Patient Concerns Philips faces further complexity with an additional investigation initiated by a Senate request for the Government Accountability Office to examine the FDA’s handling of the recall.
In December 2023, the company agreed to a $479 million class action settlement to compensate individuals who purchased, leased, or rented one of its recalled CPAP machines since 2021.
While Philips maintains its financial targets for 2025, it recognizes the necessity of addressing the aftermath of the CPAP recall.
Financial analysts view Philips’ decision to cease selling sleep apnea machines in the U.S. as strategic as the company strives to recover from one of the most extensive recalls in recent memory.