For patients and medical professionals alike, the safety of medical devices is paramount. Exactech, a well-known company in the orthopedic device market, has come under significant scrutiny in recent years. This article will explore the regulatory involvement concerning Exactech products, providing a comprehensive understanding of the steps taken to ensure these devices’ safety and efficacy. This issue is crucial for anyone involved in the use of medical implants, whether directly as patients or indirectly as healthcare providers or legal advisors.
Regulatory bodies like the U.S. Food and Drug Administration (FDA) play a critical role in monitoring and regulating medical devices. In the case of Exactech, various concerns have prompted investigations and recalls. These actions are essential to ensure that the devices meet safety standards and do not pose undue risks to patients. Exactech’s involvement with regulatory bodies highlights the importance of stringent oversight in the medical device industry.
The Role Of The FDA In Monitoring Medical Devices
The FDA is the primary regulatory body overseeing medical devices in the United States. Its role includes the evaluation, approval, and monitoring of devices like those produced by Exactech. The FDA ensures that these devices meet safety and efficacy standards before they are marketed. This process involves rigorous testing and clinical trials to identify potential risks and benefits. Key factors such as patient outcomes and the performance of components like hip and knee implants are thoroughly examined during this phase.
Once a device is on the market, the FDA continues to monitor its performance through various means, including mandatory reporting of adverse events and routine inspections. In cases where significant risks are identified, the FDA can issue recalls or safety alerts to protect public health. This ongoing surveillance is crucial for maintaining the trust and safety of medical device users, particularly for devices prone to lead to implant failure or requiring corrective revision surgery.
Exactech’s products, like many others, have undergone this extensive regulatory process. However, despite these measures, issues can still arise post-approval, necessitating further regulatory involvement. The FDA’s role in these scenarios is to act swiftly to identify and mitigate risks and ensure patient safety. For instance, the FDA’s involvement extends to monitoring potential issues like defective packaging or defective bags that can compromise the integrity of the implants.
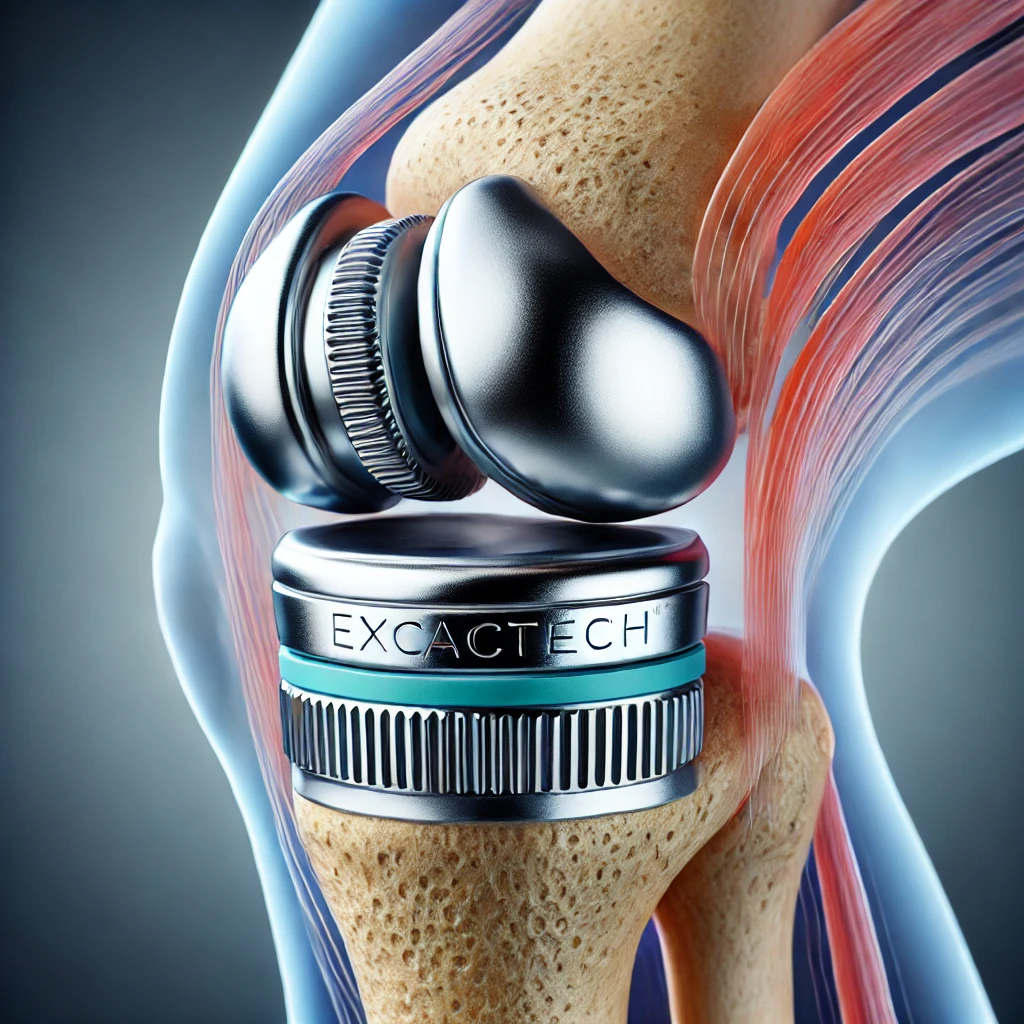
The FDA is the primary regulatory body overseeing medical devices in the United States, including those produced by Exactech Inc. Its role involves evaluating, approving, and continuously monitoring devices such as the Exactech knee system and Optetrak devices. These devices undergo rigorous testing and clinical trials to ensure safety and efficacy before they reach the market. Despite these precautions, issues can arise post-approval, leading to the risk of serious injuries and necessitating recalls. For instance, the Exactech recall for certain types of knee implants highlighted significant concerns. The root cause of these issues often relates to defects in plastic components or improper procedures. These problems have affected many patients, including veterans receiving joint replacement surgeries through Veterans Affairs facilities.
Once on the market, the FDA continues to monitor the performance of Exactech implants through mandatory adverse event reporting and routine inspections. When significant risks are identified, the FDA can issue safety alerts or recalls to protect public health. In cases involving severe complications, such as a left knee implant failure leading to serious injury, the FDA’s swift action is crucial. Additionally, regulatory actions often involve federal court proceedings, as seen in whistleblower lawsuits against Exactech Inc. These lawsuits, the whistleblower case sometimes involving co-founder Bill Petty, seek accountability for the company’s failures. The FDA’s role extends to ensuring shared decision-making between healthcare providers and patients, as well as overseeing the removal and replacement of defective implants to improve patient outcomes.
Recent Regulatory Actions On Exactech Products
In recent years, Exactech has faced several regulatory actions due to concerns over its orthopedic devices. These actions have primarily been driven by reports of device failures and associated health risks. For example, Exactech’s knee and ankle implants have been subject to recalls due to issues like premature wear and tear, which can lead to severe complications for patients, such as increased pain and the need for additional surgery. The Exactech recall for certain types of knee implants was prompted by significant issues with the plastic components. Many patients, including veterans receiving joint replacement surgeries through Veterans Affairs facilities, have experienced poor results from implant only, necessitating corrective revision surgeries and further complicating their healthcare journeys.
These recalls are a direct result of the FDA’s vigilant monitoring and the mandatory reporting system that captures adverse events. When a pattern of issues is identified, the FDA steps in to assess the situation and take appropriate action. For Exactech, this has meant multiple recalls and increased scrutiny of their manufacturing processes and quality control measures. Products like the Optetrak implant and other knee systems have been closely examined for potential risks. The root cause of these problems has often been traced back to manufacturing defects and inadequate quality control procedures. Regulatory actions have also involved federal court proceedings, including whistleblower lawsuits against Exactech Inc. and its co-founder Bill Petty, a whistleblower lawsuit aiming to hold the company accountable.
The company’s response to these regulatory actions is also crucial for patient well. Exactech has had to cooperate closely with the FDA, providing detailed information about their products and working on corrective actions. This collaboration is vital for resolving issues and preventing future occurrences, thereby safeguarding patient health. Regulatory authorities may also impose liability on the company if they fail to address these concerns adequately. Shared decision-making between healthcare providers and patients has become more critical than ever, as they navigate the complexities of choosing alternative treatments and technologies. Insights from international data, such as the Australian registry, have prompted further investigations into the risks associated with Exactech implants. The involvement of major stakeholders, like TPG Capital, and the examination of systemic issues within Exactech aim to ensure that patient health is not compromised and that future devices meet rigorous safety standards.
Impact On Patients And Healthcare Providers
The impact of Exactech implants, particularly the Optetrak finned devices, on patients and healthcare providers is profound. Many patients implanted with these implanted devices have experienced severe complications, including soft tissue damage that necessitates additional surgeries to replace the faulty implants. These complications not only affect the physical health of patients but also their skin and life quality, leading to significant emotional and financial burdens. Healthcare providers, including orthopedic surgeons, must navigate the complexities of managing these issues while ensuring patient safety. This often involves coordinating with Exactech lawyers and other legal professionals to address the ramifications of defective implants and secure appropriate remedies for affected individuals. The involvement of healthcare providers is crucial in monitoring patients, implementing corrective measures, and supporting them through their recovery process, highlighting the intricate interplay between medical care and legal advocacy in addressing such widespread health care challenges.
The regulatory involvement with Exactech products significantly impacts both patients and healthcare providers. For patients, the recalls and safety alerts can cause considerable anxiety and disruption. Those with affected Exactech knee systems or other implants may need additional surgeries or medical interventions, leading to physical pain, emotional, and financial burdens. The need for corrective revision surgery can be particularly daunting, especially when daily knee pain and swelling persist despite previous interventions. Many patients, including veterans receiving joint replacement surgeries through Veterans Affairs facilities, have faced serious injuries due to defects in Exactech implants, such as the Optetrak devices and other implants with problematic plastic components.
Healthcare providers, including surgeons and hospitals, also face challenges. They must stay informed about the latest regulatory updates and recalls to ensure patient safety. This responsibility includes monitoring patients with existing implants and advising them on the best course of action. Furthermore, providers and surgeons must navigate the logistical complexities of managing recalls, such as returning defective devices and obtaining replacements. Orthopedic surgeons play a crucial role in ensuring patients receive the appropriate treatment. Additionally, regulatory actions often involve federal court proceedings, including whistleblower lawsuits against Exactech Inc. and its co-founder Bill Petty, which seek accountability for the company’s failures. Effective communication channels between regulatory bodies, manufacturers, and healthcare providers are essential for timely action and risk mitigation.
The situation underscores the importance of robust communication channels between regulatory bodies, manufacturers, and healthcare providers. Effective communication ensures that all parties are informed and can take timely actions to mitigate risks and protect patient health. This collaborative approach is vital for managing the complexities associated with the use of medical devices, including knee implants and other technologies with similar risks. Insights from international data, such as the Australian registry, and understanding the implications of new technologies and procedures, further help in managing the fallout from these recalls. The involvement of major stakeholders, like TPG Capital, and the consideration of broader factors related to patient outcomes and healthcare, play a critical role in addressing these challenges.
How Can Mass Tort America Help You?
When facing issues with medical devices like those from Exactech, having experienced legal support is essential. Mass Tort America is well-equipped to handle these complex cases, offering comprehensive legal assistance to individuals affected by defective medical devices. We understand the intricacies of regulatory processes and can help you navigate the legal landscape to seek compensation for any harm suffered.
Choosing Mass Tort America means you benefit from a team dedicated to fighting for your rights. We provide personalized attention and have a Concierge Team to coordinate everything for clients nationwide. Whether you’re dealing with health care complications or financial burdens due to a recalled device, our legal experts are here to help. Contact us today at 800-356-4338 or visit our contact form at https://masstortamerica.com/contact/ to learn more about how we can assist you.