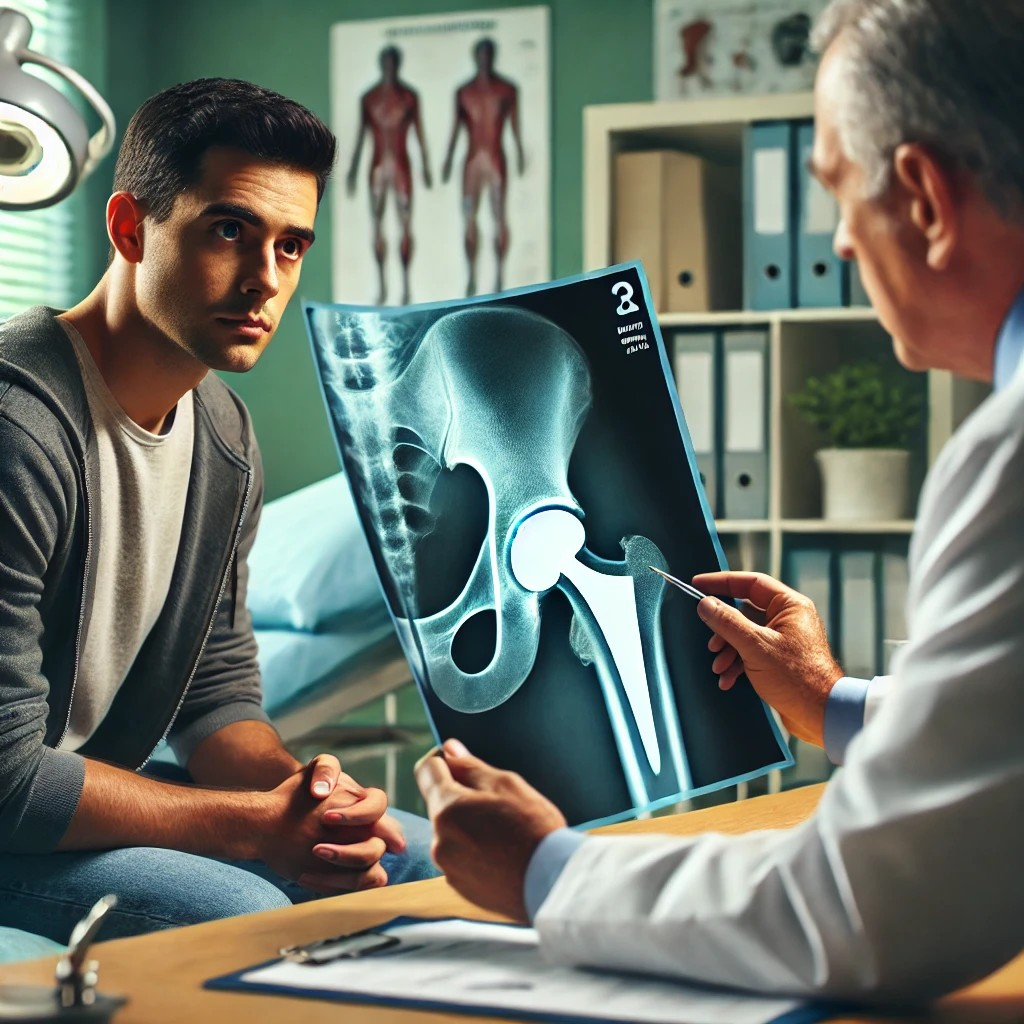
Imagine being told that your first ever hip replacement or knee replacement surgery, which was supposed to improve your quality of life, is now causing more harm than good. This is the troubling reality for many patients who received Exactech hip implants. Recently, the FDA has issued a warning regarding the accelerated degeneration of these implants, raising serious concerns about their safety and efficacy. This announcement has left many patients and their families grappling with the potential health implications and seeking guidance on what steps to take next.
At Mass Tort America, we understand the profound impact such news can have on your life. Our mission is to provide clear, reliable information and legal support to those affected by defective medical devices. With a dedicated Concierge Team to help coordinate everything, we are committed to assisting patients from all over the nation in navigating the complex legal landscape to secure the justice and compensation they deserve.
Understanding The FDA Warning
The FDA’s recent warning about Exactech hip implants centers on their unexpected accelerated degeneration. This means that the implants are wearing out much faster than anticipated, leading to severe complications for patients. Common symptoms reported include increased hip pain, inflammation, and in some cases, the need for revision or replacement surgery. This rapid wear and tear can result in particles from the implant entering the surrounding tissue, causing further damage and increasing the risk of infections and other health issues.
This issue primarily affects the polyethylene components of the new hip implants. Polyethylene is a type of plastic used in many medical devices due to its durability and compatibility with the human body. However, in the case of Exactech’ new hip implants, the material has not performed as expected. The FDA’s investigation revealed that the polyethylene liners used in these implants may degrade more rapidly than those in similar products, leading to the observed complications.
The FDA’s warning underscores the importance of regular monitoring for patients with these implants. They advise patients experiencing any unusual symptoms to seek medical attention promptly. Physicians are also urged to be vigilant in tracking their patients’ progress and to consider alternative treatment options if signs of accelerated degeneration are detected.
Implications For Patients
For patients, the FDA’s warning brings significant concerns and potential health risks. Those with Exactech hip implants are now faced with the possibility of their devices failing prematurely. This can lead to a range of serious complications, including chronic hip pain, reduced mobility, and the necessity for additional surgeries. The physical and emotional toll of these issues can be substantial, affecting not just the patients but their families as well.
The prospect of revision surgery is particularly daunting. Revision procedures are typically more complex and riskier than the initial total hip replacement surgery. They involve removing the faulty implant and replacing it with a new one, often requiring longer recovery times and increased chances of complications. For many patients, the thought of undergoing another major surgical procedure is overwhelming, especially when they had hoped the initial total hip replacement surgery would be a lasting solution.
Financial implications cannot be overlooked either. Additional medical procedures mean higher medical bills, which can be a burden for many families. Insurance may not always cover these costs fully, leaving patients and their families to manage the financial strain on top of their health concerns. This is where legal action becomes a crucial avenue for those affected, seeking compensation for the damages and financial losses incurred.
Legal Recourse And Compensation
Patients affected by the accelerated degeneration of Exactech hip implants have legal options available to them. Filing a product liability lawsuit against the manufacturer can help recover damages for medical expenses, pain and suffering, and other related costs. These lawsuits typically fall under the category of product liability claims, which hold manufacturers accountable for releasing defective products into the market.
In product liability cases, it is essential to demonstrate that the particular product was inherently defective and that this defect directly caused the plaintiff’s injury. Legal teams specializing in product liability law will gather medical records, expert testimonies, and other relevant evidence to build a strong case. The goal is to prove that the manufacturer failed to ensure the safety and reliability of their product, leading to the patient’s suffering.
Class action lawsuits are another potential legal pathway. These involve a group of individuals collectively bringing a case against the manufacturer. This approach can be particularly powerful, as it combines the resources and testimonies of multiple plaintiffs, creating a stronger case against the defendant. Class actions can also lead to more substantial settlements or verdicts, providing broader compensation for all affected parties.
Symptoms Of Accelerated Degeneration
Patients with Exactech hip implants may experience various symptoms indicating accelerated degeneration. These symptoms often include persistent pain in the hip or groin area, which can significantly impair mobility muscle strength and quality of life. As the implant deteriorates, inflammation and swelling around the hip joint may also occur, leading to discomfort and potential infections.
Another common symptom is a noticeable decrease in the range of motion. Patients might find it challenging to perform everyday activities, such as walking or climbing stairs, due to the stiffness and discomfort caused by the failing implant. In severe cases, patients may experience pain and may even hear or feel the implant grinding or clicking within the joint, which is a clear sign that the device is not functioning correctly.
Early detection of these symptoms is crucial for managing the complications associated with accelerated degeneration. Patients experiencing any of these signs should seek medical attention immediately. Timely intervention can help prevent further damage damaged bone, and improve the chances of successful treatment, whether through non-surgical methods or revision surgery.
The Role Of Medical Professionals
Medical professionals play a vital role in identifying and managing the issues related to Exactech hip implants. Orthopaedic surgeons and primary care physicians must be aware of the potential for accelerated degeneration and remain vigilant in monitoring their patients. Regular check-ups and imaging studies, such as X-rays or MRIs, are essential tools for detecting early signs of implant failure.
When patients present with symptoms indicative of accelerated degeneration, healthcare providers should conduct thorough evaluations to determine the cause. This may involve physical examinations, reviewing the patient’s medical history, and ordering diagnostic tests. Early diagnosis can lead to more effective treatment plans, reducing the risk of severe complications and improving patient outcomes.
Physicians must also educate their patients about the risks associated with Exactech hip implants. Providing clear and comprehensive information about the potential for accelerated degeneration can help patients make informed decisions about their treatment options. Additionally, physicians should discuss the importance of regular follow-up appointments to monitor the condition of the implant over time.
Preventive Measures And Monitoring
Preventive measures and regular monitoring are essential for patients with Exactech hip implants to mitigate the risks of accelerated degeneration. Patients should adhere to their follow-up schedule with their healthcare providers, ensuring that any changes in the condition of the implant are detected early. Regular imaging studies can help monitor the integrity of the implant and identify any signs of wear and tear.
In addition to regular check-ups, patients can take proactive steps to maintain the health of their hip implant. Maintaining a healthy weight can reduce the stress on the hip joint and decrease the likelihood of complications. Patients should also avoid high-impact activities that may accelerate the wear of the implant, such as running or jumping, and opt for low-impact exercises like swimming or cycling.
Patients should be aware of the importance of reporting any new or worsening symptoms to their healthcare providers promptly. Early intervention is key to managing complications and preventing further damage. By staying vigilant and proactive in their care, patients can improve their chances of maintaining a functional and pain-free first after hip replacement surgery, or joint replacement.
Impact On Quality Of Life
The accelerated degeneration of Exactech hip implants can have a profound impact on patients’ quality of life. Chronic hip pain and reduced mobility can significantly impair daily activities and overall well-being. Patients may struggle with simple tasks such as walking, getting in and out of chairs, or even sleeping comfortably, leading to a diminished quality of life.
The physical therapist emotional toll of dealing with a failing hip implant can also be substantial. Patients may experience anxiety, frustration, and depression as they cope with ongoing pain and the prospect of additional surgeries. The uncertainty surrounding their health and the potential for long-term complications can be mentally and emotionally draining for both physical therapy patients and their families.
Addressing these quality-of-life issues requires a comprehensive approach that includes medical treatment, pain management, physical therapy, and emotional support. Patients should collaborate with their healthcare providers to create a tailored treatment plan that addresses both the physical and emotional aspects of their condition. Access to mental health resources and support groups can also be beneficial for coping with the challenges associated with implant failure.
The Role Of Manufacturers
Manufacturers of medical devices like Exactech have a significant responsibility to ensure the safety and effectiveness of their products. This involves rigorous testing and quality control measures during the design and manufacturing process. However, the accelerated degeneration of Exactech hip implants indicates potential shortcomings in these processes, raising questions about the company’s oversight and accountability.
When issues with a medical device arise, manufacturers are obligated to investigate the problem thoroughly and take appropriate action to protect patients. This may include issuing recalls, providing updated safety information to healthcare providers, and developing solutions to address the defect. Transparent communication with regulatory authorities and the public is essential to maintain trust and ensure patient safety.
In the case of Exactech hip implants, the company’s response to the FDA’s warning will be closely scrutinized. Patients and their families will be looking for assurance that the manufacturer is taking the necessary steps to rectify the issue and prevent further harm. Accountability and proactive measures are critical for restoring confidence in the company’s products and practices.
The Importance Of Regulatory Oversight
Regulatory oversight is crucial in ensuring the safety and efficacy of medical devices. Agencies like the FDA play a vital role in monitoring the performance of medical products and protecting public health. Through stringent approval processes, post-market surveillance, and timely warnings, regulatory bodies help identify and address potential issues before they become widespread.
The FDA’s warning about Exactech hip implants highlights the importance of continuous monitoring and vigilance. By analyzing data from adverse event reports, clinical studies, and other sources, the FDA can identify patterns and trends that indicate potential problems. This proactive approach allows for timely intervention and helps prevent further harm to patients.
Regulatory oversight also involves collaboration with manufacturers, healthcare providers, and patients to improve the safety of medical devices. By working together, these stakeholders can develop better products, enhance patient education, and implement more effective monitoring systems. Continuous improvement and innovation are essential to advancing the field of medical devices and ensuring patient safety.
Steps For Affected Patients
Patients affected by the accelerated degeneration of Exactech hip implants should take several important steps to address their situation. First and foremost, it is essential to seek medical attention if you are experiencing any symptoms associated with implant failure. Your healthcare provider can conduct a thorough evaluation and determine the appropriate course of action.
Keeping detailed records of your medical history, symptoms, and treatments can be valuable for both medical and legal purposes. Documenting your experiences can help your healthcare provider make informed decisions about your care and support any potential legal claims you may pursue. These records can provide crucial evidence in proving the impact of the defective implant on your health and well-being.
If you are considering legal action, consulting with a qualified attorney who specializes in product liability cases is advisable. An experienced legal team can guide you through the process, help you understand your rights, and work to secure compensation for your medical expenses, pain and suffering, and other related damages. Taking prompt action can improve your chances of achieving a favorable outcome.
Long-Term Health Considerations
For most patients, with Exactech hip implants, long-term health considerations are a significant concern. The accelerated degeneration of these implants can lead to ongoing health issues that may require continuous management. Chronic pain, limited mobility, and the potential for multiple revision surgeries are just a few of the long-term challenges patients may face.
Ongoing medical care and monitoring are essential for managing these long-term health issues. Patients should keep regular follow-up appointments with their healthcare providers to monitor their implants’ condition and promptly address any new symptoms. A proactive approach to health management can help mitigate the impact of implant failure and improve overall outcomes.
Patients should also consider the potential need for lifestyle adjustments to accommodate their condition. This may involve modifying daily activities, adopting a healthier diet, and engaging in low-impact exercises to maintain joint health and mobility. By making these adjustments, patients can improve their quality of life, relieve pain, and reduce the risk of further complications.
Patient Advocacy And Support
Patient advocacy and support play a crucial role in addressing the challenges faced by those affected serious injuries done by defective medical devices like Exactech hip implants. Advocacy groups and support organizations provide valuable resources, information, and assistance to patients navigating the complexities of their condition. These organizations can offer guidance on medical treatment options, legal rights, and emotional support.
Connecting with others who have experienced similar issues can be incredibly beneficial for patients. Support groups and online communities provide a platform for sharing experiences, advice, and encouragement. These connections can help patients feel less isolated and more empowered to manage their health and pursue legal action if necessary.
Patient advocacy also involves raising awareness about the issues associated with defective medical devices. By highlighting the experiences of affected individuals and advocating for stricter regulations and oversight, these organizations can drive change and improve the safety and effectiveness of medical products. Collective action can lead to significant improvements in patient care and outcomes.
The Future Of Hip Implant Technology
The challenges associated with Exactech hip implants underscore the need for continuous innovation and improvement in the hip joint implant technology. Advances in materials science, design, and manufacturing processes can lead to the development of more durable and reliable implants. Research and development efforts are focused on creating implants that better mimic the natural biomechanics of the hip joint and reduce the risk of complications.
Emerging technologies, such as 3D printing and customized implants, offer promising solutions for improving the fit and performance of hip replacements. These technologies allow for more precise tailoring of implants to individual patients, potentially reducing the risk of wear and failure. Continued investment in research and collaboration between medical professionals, engineers, and manufacturers are essential for driving these advancements.
The future of hip implant technology also involves enhancing the monitoring and diagnostic tools available to healthcare providers. Improved imaging techniques, wearable devices, and data analytics can provide real-time insights into the condition of implants and help detect issues earlier. By integrating these technologies into patient care, the medical community can improve outcomes and reduce the incidence of complications associated with hip implants.
Manufacturing Defects And Quality Control
The issue with Exactech hip implants raises significant concerns about manufacturing defects and quality control. During the manufacturing process, any lapses in quality control can lead to defects that compromise the integrity and performance of the implants. Such defects may not always be immediately apparent and can result in long-term complications for patients.
Manufacturing defects can include issues with the materials used, such as the polyethylene liners, or problems with the assembly line process. These defects can accelerate the wear and tear of the implant, causing pain and leading to the need for revision surgery. Ensuring stringent quality control measures during production is crucial to prevent these issues from arising.
To address manufacturing defects, it is essential for manufacturers to implement comprehensive quality assurance programs. This includes regular inspections, testing of materials, and adherence to industry standards. By maintaining high-quality control standards, manufacturers can reduce the risk of defective products reaching patients and ensure the safety and efficacy of their medical devices.
Design Defects And Product Liability
Design defects are another critical aspect of the problems associated with Exactech hip implants. A design defect occurs when the design of a product is inherently unsafe, even if manufactured correctly. In the case of Exactech hip implants, the design of the polyethylene liners may contribute to their accelerated degeneration, causing harm to patients.
Product liability laws hold the product manufacturers more accountable for design defects that result in injury or harm. Legal action can be taken under strict, product liability claims, where the focus product liability claim is on the product’s design rather than the manufacturing process. Demonstrating that the design was flawed and caused the plaintiff’s injury is crucial in these cases.
To prevent design defects, manufacturers must invest in thorough research and development. This involves extensive testing and validation of designs to ensure they meet safety and performance standards. By prioritizing safety in the design phase, manufacturers can create more reliable products and minimize the risk of design-related failures.
Legal Theories In Product Liability Cases
Product liability cases can be based on various legal theories of product defects, including strict product liability claim only, negligence, and breach of warranty. Strict liability focuses on the product’s condition, regardless of the manufacturer’s intent or negligence. In strict products liability cases, plaintiffs must prove that the product was defective and caused their injury.
Negligence involves demonstrating that the manufacturer failed to exercise reasonable care during the design, manufacturing, or distribution of the defective product.. This could include lapses in quality control or failure to conduct adequate testing. Plaintiffs must show that the manufacturer’s negligence directly led to the defect and their subsequent injury.
Breach of warranty claims can be based on an express or implied warranty; express warranties, which are explicit promises made by the manufacturer, or implied warranties, which are unspoken guarantees that the product will perform as expected. When a product fails to meet these warranties, affected consumers may pursue legal action to seek compensation for damages.
Evaluating The Consumer Expectation Test
The consumer expectation test is a legal standard used in product liability cases to determine whether a product is unreasonably dangerous. Under this test, a product is considered defective if it fails to perform as safely as an ordinary consumer would expect when used in a reasonably foreseeable manner. This standard applies to both design and manufacturing defects.
In the context of Exactech hip implants, the consumer expectation test can be used to evaluate whether the implants met the reasonable expectations of patients. If the implants failed prematurely and caused harm, they may be considered defective under this test. Legal teams will gather evidence to demonstrate that the implants did not meet the safety expectations of patients and healthcare providers.
This test is particularly relevant in cases where the defect is not immediately apparent or where the product’s failure is due to complex technical issues. By focusing on the consumer’s reasonable expectations, this standard provides a practical approach to evaluating product safety and holding manufacturers accountable.
Assessing The Risk Utility Test
The risk utility test is another standard used in product liability cases to assess whether a product’s design is defective. This test involves weighing the defective product”s risks against its utility or benefits. A product may be considered defective if the risks posed by its design outweigh its benefits to consumers.
For Exactech total hip replacement implants, the risk utility test can be applied to evaluate whether the design of the implants provided sufficient benefits to justify the risks of accelerated degeneration. Legal teams will analyze factors such as the likelihood and severity of potential injuries, the feasibility of alternative designs, and the overall benefits provided by the implants.
This test requires a comprehensive evaluation of the product’s design, considering both the technical aspects and the real-world impact on patients. By thoroughly assessing the risks and benefits, legal teams can build a compelling case to demonstrate that the design of the implants was unreasonably dangerous and led to patient harm.
Navigating The Legal Process
Navigating the legal process in product liability cases can be complex and challenging. Patients affected by defective products like Exactech hip implants should seek legal representation from experienced attorneys who specialize in product liability law. These legal professionals can guide clients through the various stages of litigation, from filing a claim to negotiating settlements or pursuing a trial.
The legal process typically begins with an initial consultation, where the attorney will assess the case and determine the best course of action. This involves gathering evidence, such as medical records, expert testimonies, and documentation of the product defect. The attorney will then file a formal complaint, outlining the plaintiff’s claims and seeking compensation for damages.
Throughout the litigation process, the attorney will represent the client’s interests, negotiating with the defendant’s legal team and presenting the case in court if necessary. Effective legal representation is crucial for achieving a favorable outcome and securing the compensation needed to cover medical expenses, lost wages, and other related costs.
The Importance Of Expert Testimony
Expert testimony plays a vital role in product liability cases involving defective medical devices like Exactech hip implants. Experts in fields such as orthopaedic surgery, materials science, and medical device manufacturing can provide critical insights into the nature of the defect and its impact on patients. Their testimonies can help establish the link between the defect and the plaintiff’s injury.
In cases involving accelerated degeneration, experts can explain how the design or manufacturing defects contributed to the premature wear of the implants. They can also discuss the medical implications of the defect, including the need for revision or replacement surgery, and the long-term health effects on patients. This expert testimony can strengthen the plaintiff’s case and provide a clear, credible explanation of the issues at hand.
Selecting qualified and reputable experts is essential for building a strong case. Legal teams will collaborate with these experts to ensure their testimonies are thorough, well-supported, and clearly presented. Expert testimony can be a decisive factor in persuading judges and juries of the validity of the plaintiff’s claims.
Medical And Legal Coordination
Effective coordination between medical and legal professionals is crucial for patients pursuing claims related to defective hip implants. Medical professionals can provide the necessary documentation and expert opinions to support the legal case, while attorneys can ensure that patients’ rights are protected and that they receive the compensation they deserve.
This coordination involves regular communication and collaboration between the hospital room the patient’s healthcare providers and legal team. Medical professionals can help document the patient’s condition, treatment history, and the impact of the defective implant on their health. This information is vital for establishing the extent of the injury and the need for compensation.
Attorneys can assist in gathering and organizing medical records, arranging for expert testimonies, and presenting the case effectively in court. By working together, medical and legal professionals can provide comprehensive support to patients, ensuring their medical needs are met while pursuing justice and compensation for their injuries.
Why Choose Mass Tort America?
At Mass Tort America, we are dedicated to helping individuals who have been harmed by defective medical devices like Exactech hip implants. Our experienced legal team understands the complexities of product liability and class action lawsuits, and we are committed to fighting for your rights. We work tirelessly to ensure that you receive the compensation you deserve, covering medical expenses, lost wages, and pain and suffering.
Choosing Mass Tort America means choosing a team that truly cares about your well-being. We offer personalized attention and support throughout the legal process, ensuring you are informed and empowered every step of the way. Our Concierge Team is here to assist you with all logistical aspects, making the entire experience as smooth and stress-free as possible. To learn more and start your journey toward justice, contact us today at 800-356-4338 or visit our contact form at https://masstortamerica.com/contact.